As 'Skinny' FDA User Fee Reauthorizations Cross Finish Line, Congress Prepares to Revisit FDA Policy Riders in Lame Duck Session

On September 30, 2022, Congress enacted legislation to reauthorize the Food and Drug Administration (FDA) user fee programs for branded/reference drugs and biologics, medical devices, generic drugs and biosimilars (PDUFA, MDUFA, GDUFA and BsUFA). Congress incorporated this five-year reauthorization into the Continuing Resolution (CR) to fund the federal government until December 16, 2022. While the legislation also includes short-term extensions for certain FDA programs that were also set to expire, Congress did not include any substantive policy riders that had previously advanced as part of the House and Senate committee action to reauthorize the FDA user fee programs (H.R. 7667, S. 4348). This alert provides an overview of the user fee reauthorization legislation and an update on other public health and FDA-related provisions that Congress may consider before the end of the year.
UFA Implementation Moves Forward
The inclusion of the five-year user fee reauthorizations in the CR is the culmination of a multiyear effort. The core of the FDA provisions in the CR are the reauthorizations of the Prescription Drug User Fee Amendments of 2017 (PDUFA), Generic Drug User Fee Amendments of 2017 (GDUFA), Biosimilar User Fee Amendments of 2017 (BsUFA) and the Medical Device User Fee Amendments of 2017 (MDUFA). These complement the commitment letters negotiated between the industry and FDA with regard to each of the four programs. The FDA, with input from industry and other stakeholders, will move forward with implementing PDUFA VII, GDUFA III, BsUFA III and MDUFA V. The FDA has not yet posted the new fee amounts that will apply beginning October 1, and the agency will likely provide guidance to the industry in the coming days about the new fee levels and how they will be implemented on short notice.
Unfinished Business: Congress Could Revisit FDA Policy Riders, Public Health Bills in Lame Duck
Notably absent from the CR’s UFA reauthorization are the provisions related to the regulation of in vitro clinical tests (the VALID Act), cosmetics and dietary supplements. These three “super riders” were included in S. 4348, the Food and Drug Administration Safety and Landmark Advancements (FDASLA) Act, which the Senate HELP Committee approved in June, but were not part of the House-passed Food and Drug Amendments of 2022 (H.R. 7667). As noted, the final legislation omitted all substantive policy changes that had been contemplated in the respective legislation of the House and the Senate. Even those areas of policy reform that were addressed in both the House and Senate legislation were omitted from the final bill, such as provisions related to accelerated approval reforms, generic drug labeling, development of rare disease endpoints and agency inspection activities. The following chart highlights how the FDA-related provisions included in the CR compare to the leading UFA reauthorization bills.
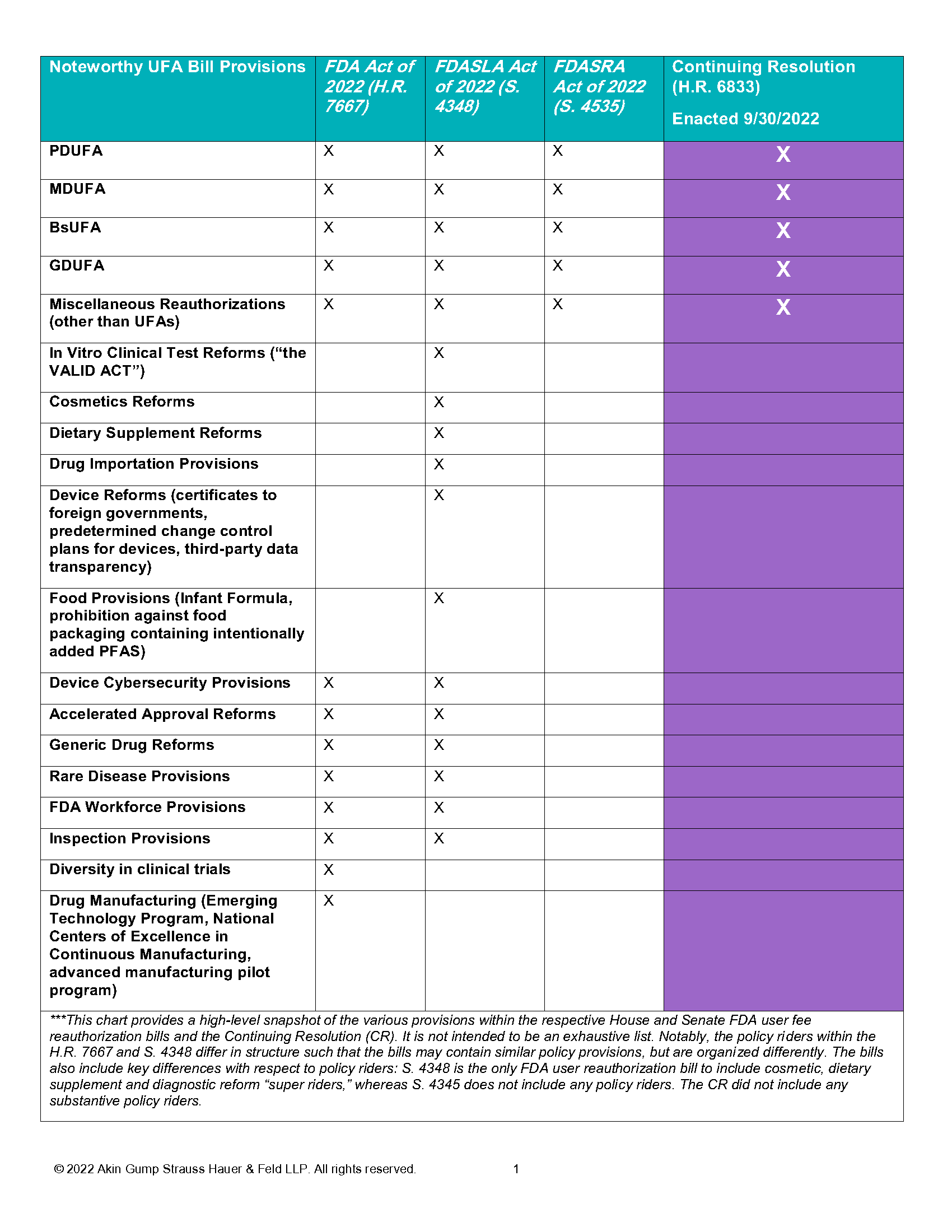
Outlook for FDA Policy Riders
While Congress elected to advance a “clean” reauthorization and did not include substantive FDA policy riders in the CR, the House Energy and Commerce Committee and Senate HELP Committee have signaled interest in continuing to deliberate on the provisions included in their respective legislative proposals, with the goal of reaching consensus on additional FDA-related legislation for consideration before the end of the year. The fact that Congress is only reauthorizing some FDA programs until December 16, 2022, dictates a need to revisit FDA legislation in December; otherwise, these programs will sunset. These provisions include reauthorizations that have had long-standing, congressional support over the years, such as reauthorization of certain device inspections, orphan drug grants and the Best Pharmaceuticals for Children Program (BPCA), among others.
Potential FDA-related legislation at the end of the year could include the HELP Committee’s proposals on diagnostics, cosmetics and dietary supplements, or other provisions included in the House or Senate packages. However, the full scope of FDA-related provisions that could be in play on such a package is not limited to provisions included in the House and Senate FDA reauthorization bills.
- S. 3799, the Prepare for and Respond to Existing Viruses, Emerging New Threats and Pandemics Act (the “PREVENT Pandemics Act”), which the HELP Committee advanced in April, is also pending. The public health and preparedness legislation includes FDA reforms related to clinical trials, advanced manufacturing, foreign inspections, medical devices and FDA workforce, among other areas. Provisions from this legislation could also be included as part of a larger end-of-year health care package.
- The “Cures 2.0” legislation released last year by Reps. Diana DeGette (D-CO) and Fred Upton (R-MI) remains pending in the Energy and Commerce Committee and includes several policies that affect the FDA. These include proposals related to digital health regulation, innovative clinical trial designs, cell and gene therapy regulation, real-world evidence and breakthrough therapies.
There also continues to be congressional interest in a range of health care issues beyond the focus on FDA-related provisions. For example, Congress continues to consider mental health legislation and telehealth flexibilities beyond the COVID-19 public health emergency, and may decide to take action to avert potential Medicare cuts or enact programmatic reforms before the end of this Congress. For example, the Clinical Laboratory Fee Schedule (CLFS) tests and services will see Medicare cuts of up to 15 percent on January 1, 2023, absent congressional intervention. Bipartisan legislation, the Saving Access to Laboratory Services Act (SALSA), has been introduced in both the House and Senate to permanently amend the Protecting Access to Medicare Act of 2014 (PAMA). Congress may decide to legislate on a range of health care issues as part of a broad, end-of-year legislative package, including those related to the FDA.
Stakeholders should closely watch developments in the weeks ahead as Congress prepares to return for an end-of-year legislative push after the midterm elections. In the interim, Congress has once again reset the UFA clock, and the FDA, industry and stakeholders will move forward with implementing PDUFA VII, MDUFA V, GDUFA III and BsUFA III.



